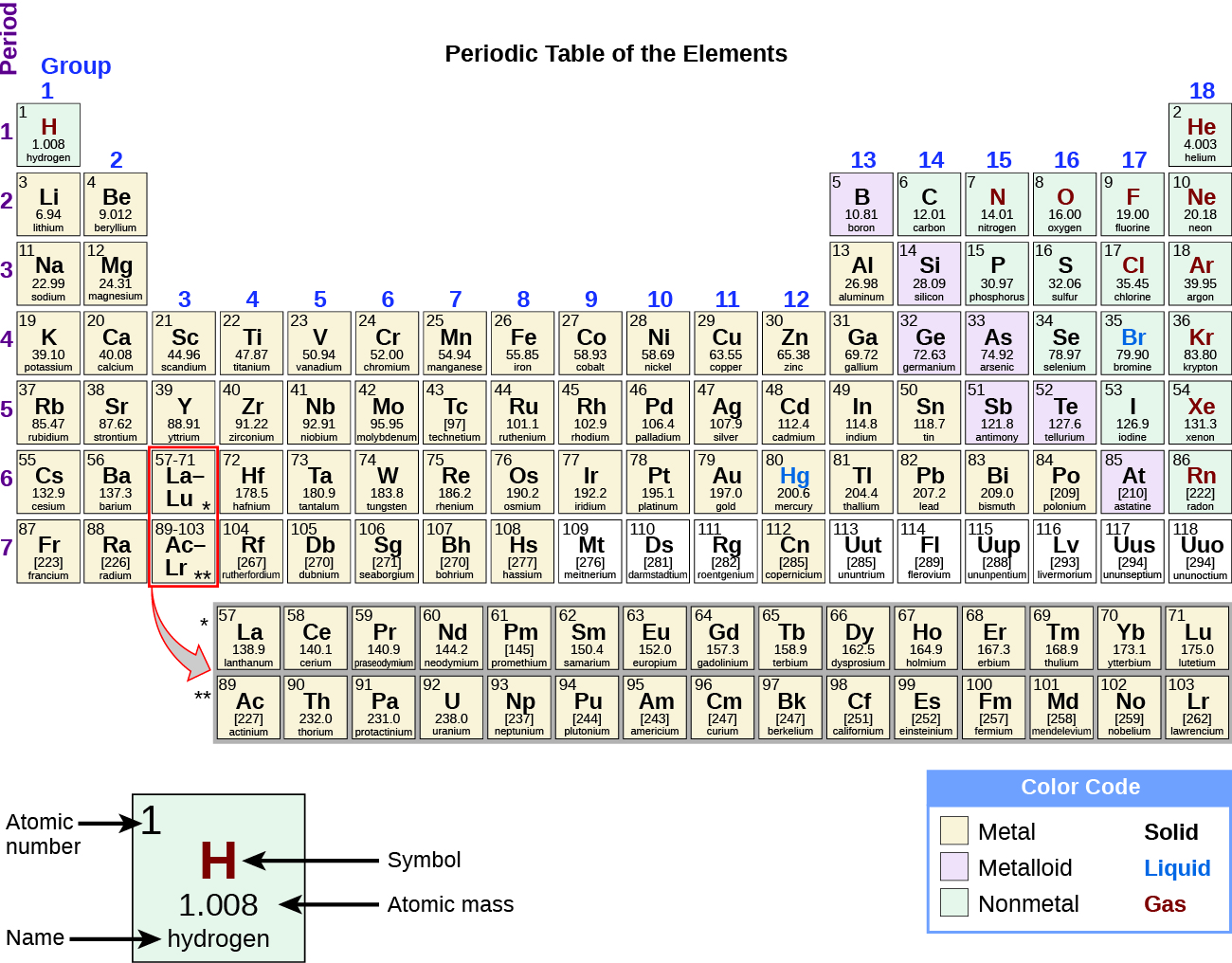
Transcript. The periodic table organizes elements into groups and periods based on their chemical and physical properties. Elements in the same group share similar characteristics, like reactivity. The table is divided into metals, nonmetals, and metalloids, each with distinct properties. Key groups include alkali metals, alkaline earth metals.. Figure 1.1.1 1.1. 1 compares the three states of matter and illustrates the differences at the molecular level. Figure 1.1.1 1.1. 1: A Diatomic Substance (O 2) in the Solid, Liquid, and Gaseous States: (a) Solid O 2 has a fixed volume and shape, and the molecules are packed tightly together. (b) Liquid O 2 conforms to the shape of its container.
The Periodic Table Chemistry
Periodic Table Of Elements Solids Liquids And Gases
Periodic Table Gases And Liquid
Solids Liquids And Gases On Periodic Table
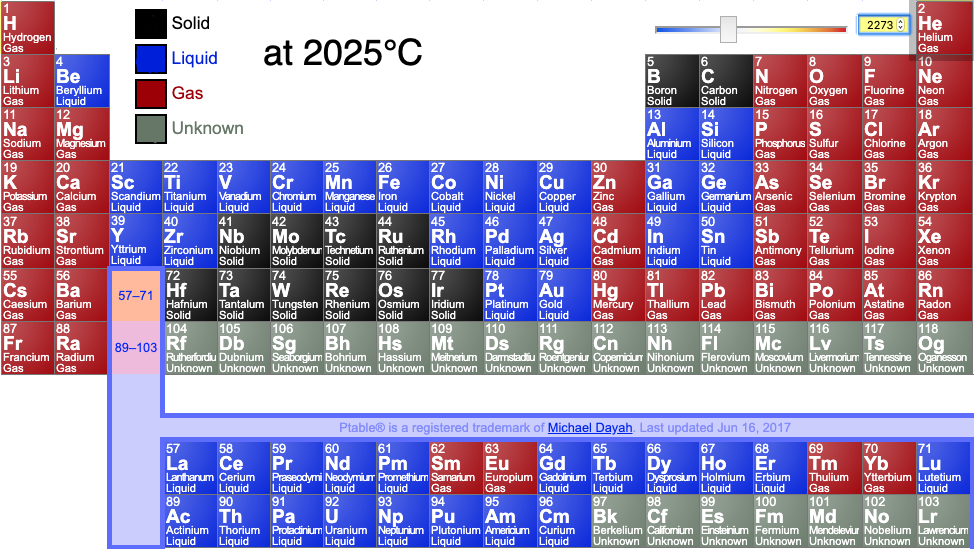
Periodic Table Of Elements With Solid Liquid And Gases

Periodic table Elements, Properties, Periodicity Britannica

Periodic Table Of The Elements Including Solid Liquid Gas And Unknown Images and Photos finder
List of all the solid elements in the periodic table INSIDE CHEMISTRY

Bi En La Tabla Periodica
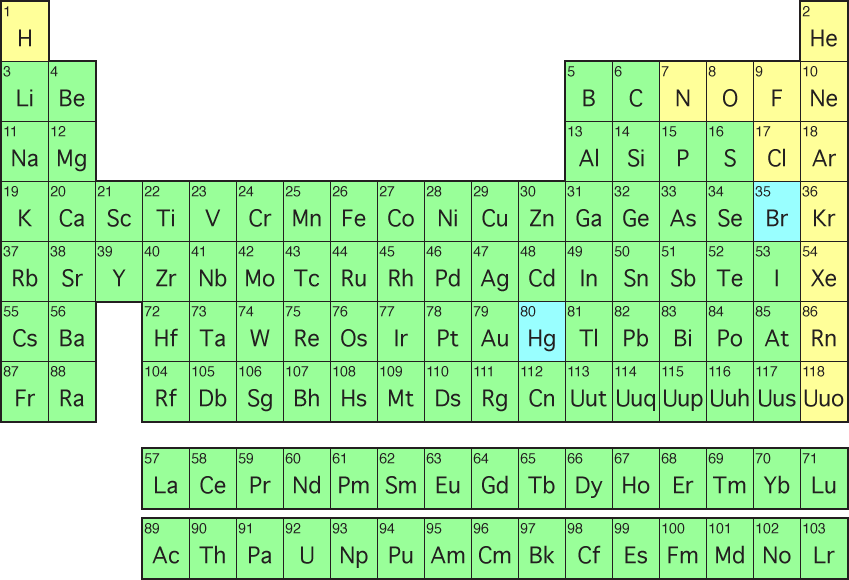
301 Moved Permanently

Periodic Table Of Elements With Solid Liquid And Gases
:max_bytes(150000):strip_icc()/Periodic-Table-Metals-56a12db33df78cf772682c44.png)
Periodic Table Of Elements With Solid Liquid And Gases

Periodic Table Of Elements Two Liquids

Periodic Table of Natural State of Elements
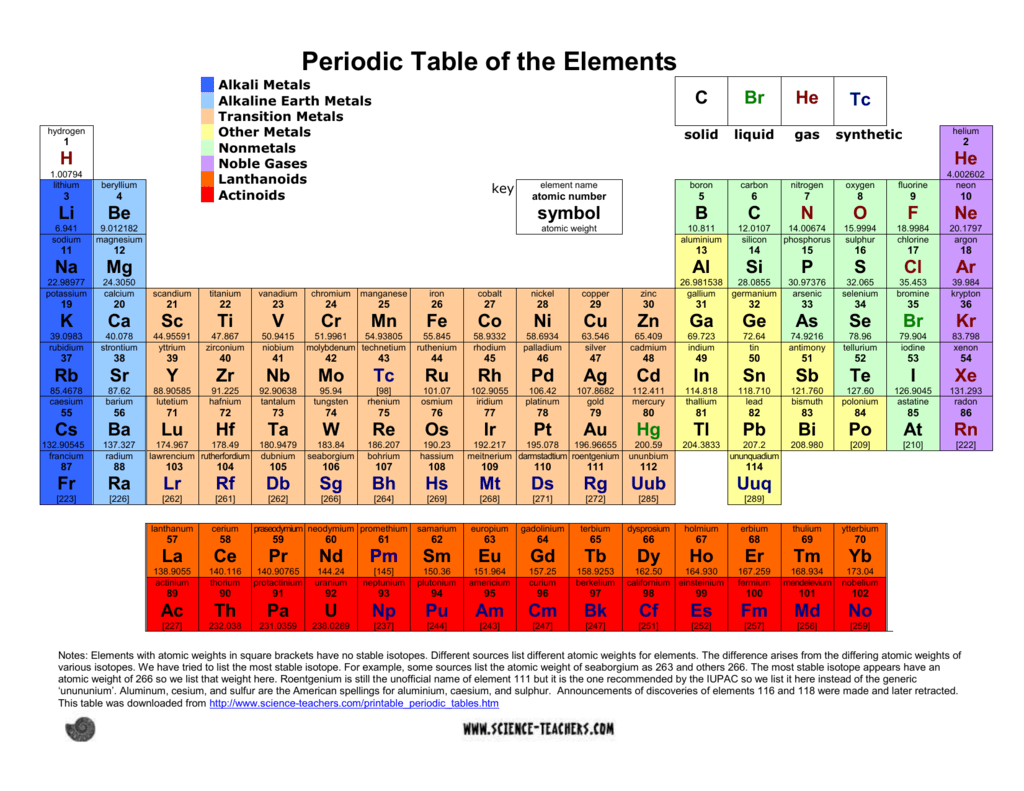
Periodic Table Showing Solids Liquids And Gases

Periodic table simple states solid liquid gas andcolader
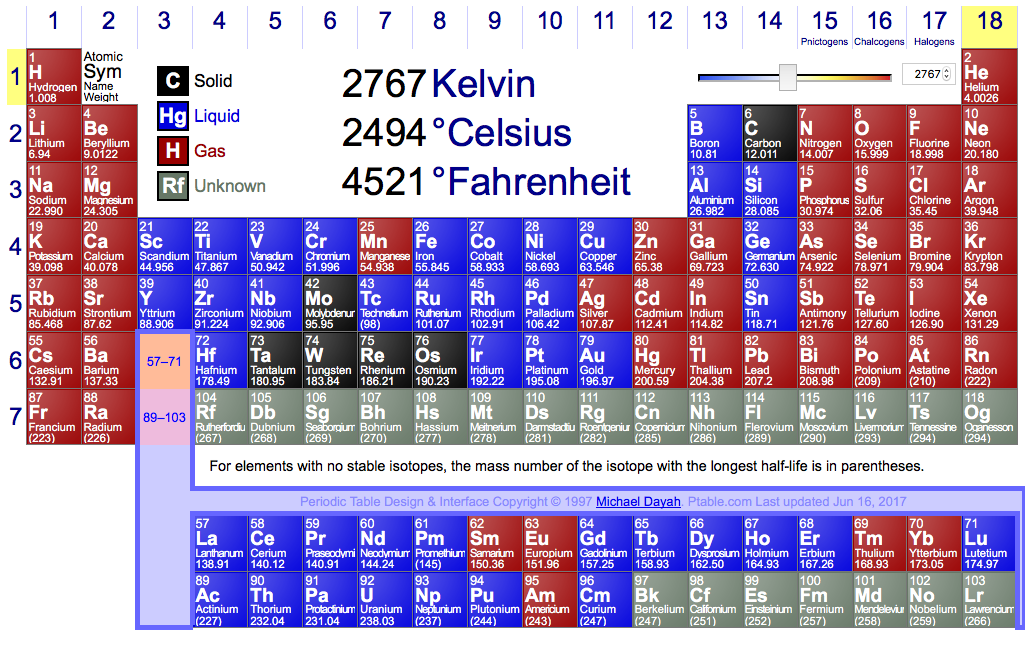
Periodic Table Of Elements Solids Liquids And Gases
Periodic Table Of Elements With Solid Liquid And Gases
Periodic Table Solids Liquids Gasses Periodic Table Timeline
Periodic Table Of Elements With Solid Liquid And Gases
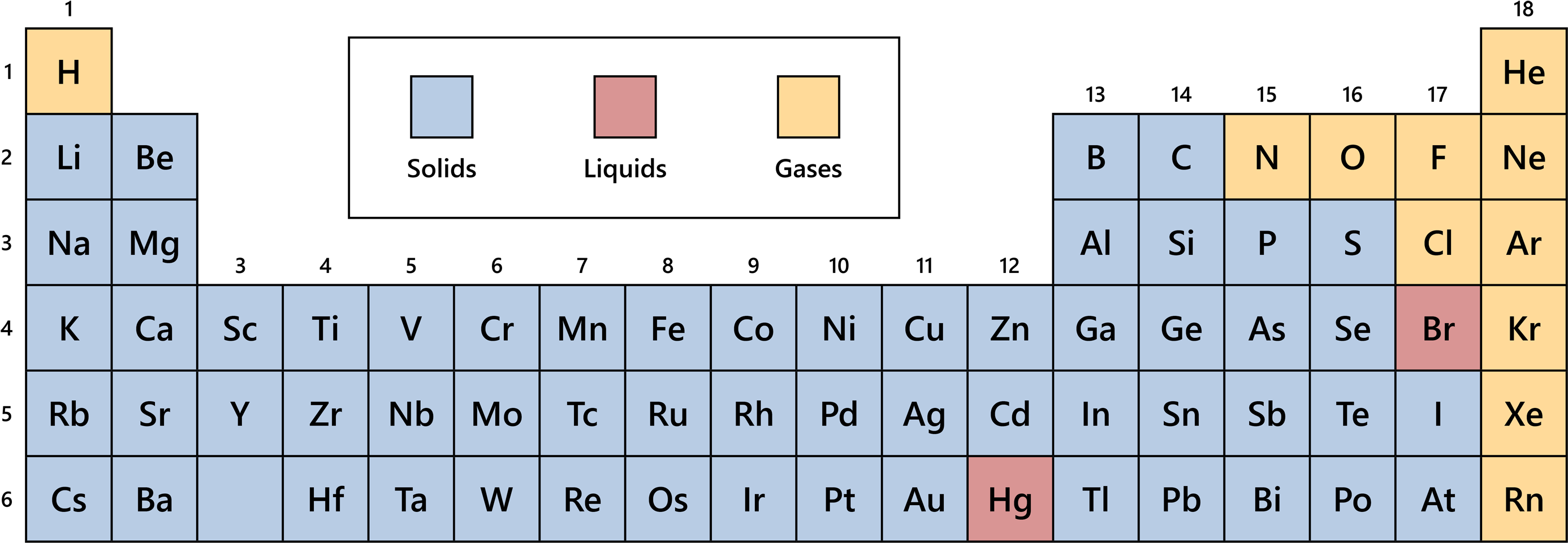
They don’t pour like a liquid. The particles vibrate back and forth within their fixed positions and do not move freely. Solids are incompressible and have high density, compared to liquids and gases. They can be crystalline, like table salt, or amorphous, like glass, rubber or plastic. Many elements exist as solid-state at room temperatures.. In the periodic table, the states of matter can be easily traced. All metals with the exception of Mercury are solids this is due to the presence of strong metallic bonding. Whilst they are all solids, some are harder than others. For example, Group 1 metals like Lithium and Sodium are soft and easy to cut. But metals such as Copper and Iron in.

